Cards In This Set
| Front | Back |
 Naive T-cells are activated by strong signaling from APCs; weak signaling or interactions with immature APCs induces T cell anergy. |
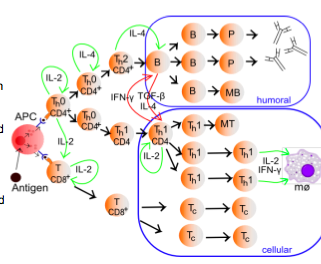
-CD4+ and CD8+ cells are stimulated by IL-2 acting as an autocrine or paracrine factor to induce proliferation and/or maturation. -expose to IL-4 drives CD4+ Th cells towards Th2 function while continues exposure to IL-2 in the absence of IL-4 drives CD4+ Th cells towards Th1 function. Th2 cells support humoral immunity by producing IL-4, 5, 10.-Th1 cells support cellular immunity by producing IL-2 and IFN-gamma. -IFNgamma produced by activated Th1 cells inhibits the activation of Th2 cells-TGFbeta produced by activated B cells inhibits the activation of Th1 cells (cross inhibition between the humoral and cellular arms)
|
|
T cell co-receptor stabilizes synapse and enhances signaling
|
-in addition to MHC-TCR interactions, co-receptors must be ligated at the immunological synapse in order to obtain a complete activation.-B7 (CD80/86) is upregulated on APC (dendritic cells and macrophages) in response to TLR stimulation-Co-binding of MHC-II/TCR with B7/CD28 on CD4+ cells results in cytokine release characteristic of Th1 or Th2 responses (Th2-IL-4 IL-5 IL-10) (Th1-IFNgamma and IL-2)-Co-binding of MHC-I/TCR with B7/CD28 on CD8+ cell results in cytokine release characteristic of Tc cells (and of Th2 cells), which leads to activation of macrophages (cellular response) and inhibition of humoral responses.
|
|
Tc cells can also be activated in the periphery without concomitant recognition between Tc TCR and MHC-I if sufficient IFN-gamma and IL-2 is generated by local Th1 cells.
|
This activation mechanism allows Tc cells with appropriate specificity (initially activated in the lymph node by APCs presenting relevant peptides)to be fully activated at sites of inflammation. Peripheral activation produces more Tc cells and/or induces Tc cells to express high levels of cytotoxic factors.-Engagement of Tc TCR and MHC-1 without co-receptor activation or without supporting cytokine signaling induces Tc anergy or Tc cell death. Apoptosis of Tc cells is initiated by engagement of CD95/CD95L (Fas/FasLigand) in the absence of CD28/B7
|
|
B cells are initially activated by antigen interactions with the BCR.
|
-Bound antigen is endocytosed and presented on MHC-II for recognition by Th2 cells expressing appropriate TCRs. -An initially activated B cell becomes fully activated by the engagement of appropriate co-receptors found on the B-cell and on the Th2 cells (eg: CD40 on the b-cell and CD40L on the Th2 cell) in the context of TCR binding to MHC-II. Full B-cell activation is also supported by the production of Th2 cytokines (IL-4,5,10)
|
|
-Signaling between B-cells and T-cells, or between APCs and T-cells, or between Tc cells and targets occurs at the immunological synapse, a structured domain of signaling molecules and adhesion molecules localized within a lipid domain enriched in specific lipids and glycolipids.
|
-development of the immunological synapse in initiated by adhesion molecules which form an adhesive plaque that connects interacting cells. Signaling molecules are subsequently recruited to the adhesive plaque, initially surrounding it and then migrating toward the center of the plaque to form a unique signaling domain. -the mature immunological synapse between APCs (including B-cells) and T-cells in characterized by a central signaling domain surrounded by adhesive proteins. The immunological synapse between Tc cells and target cells is characterized by a central signaling domain adjacent to a membrane patch specialized for fusion of lytic granules; both the signaling domain and the secretory domain are surrounded by adhesive proteins.
|
|
The purpose of the immunological synapse is to provide a stable platform for for extended signaling between cells. Activation of T-cells, B-cells, and the killing of target cells by Tc cells is a gradual process.
|
-Recruitment of appropriate receptors and co-receptors to the synapse ensures that the immune cells have multiple opportunities to abort the activation/killing process. -Interactions between APCs and T-cell or between Th2 cells and B-cells can take days to build to full activation, depending on cytokine and receptor expression levels.
|
|
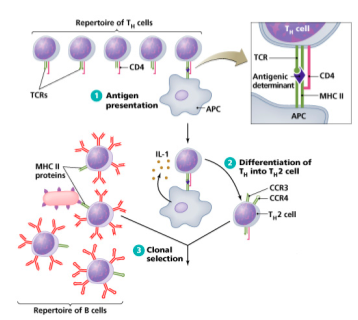
In an integrated immune response, coordinated activation of humoral and cellular immunity to the same pathogen challenge arises from clonal selection of B-cells that express BCRs which bind antigens containing peptides capable of activating TCR signaling on Th1 and Th2 cells.
|
 |
|
Example of coordinated T/B cell response: -Antigen A, derived from a specific pathogen, initiated the activation of B-cells expressing BCRs that recognize Antigen A. -Antigen A is also endocytosed into APCs and presented on MHC-II to Th cells that express TCRs which bind peptides derived from Antigen A. -Activated Th2 cells support the continued activation of B-cells that recognize Antigen A since these activated B-cells present peptides on MHC-II that are recognized by Th2 TCRs at an immunological synapse, eventually leading to the cytokines that stimulate macrophage and granulocyte attack on pathogen. So, the same pathogen induces B-cell activation and Th cell activation on cells that possess BCR or TCRs with overlapping binding specificity.
|
Taking this example further, APCs also activate Th1 cells that support the differentiation of Tc cells expressing TCRs capable of recognizing peptides derived from antigen A presented in the context of MHC-I. Therefore, Tc activation is most perinent to viral infection of intracellular pathogens in which viral or pathogen proteins are presented on infected cells, leading to the formation of an immunological synapse between Tc and the target cell.
|
|
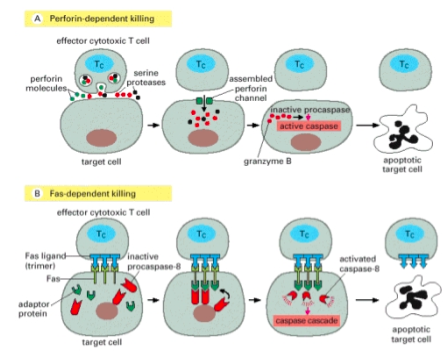
Activated Tc cells engage target cells through TCR/MHC-I interactions and kill by two mechanisms, both of which converge on apoptosis.
|
1. Perforin-dependent: lytic vesicles fuse at the secretory site of the immune synapse to release perforin and granzymes. Perforin is a pore-forming protein that allows granzymes to enter the target cell. Granzymes are serine proteases that cleave pro-caspases to their active, apoptosis-inducing forms.1. Fas-dependent target cell death: activated Tc also express CD95L (Fas ligand) on their surface which can engage with CD95 on target cells to induce apoptosis through direct caspase activation.
|
|
Perforing-dependent and Fas-dependent Target cell death
|
 |
|
The killing potential of Tc cells is enhanced by the direct action of cytokines on target cells.
|
TNFalpha and INF-gamma are released by activated Tc cells.-TNFalpha binds to target cells expressing the TNFalpha receptor and induces cells death. -INF-gamma upregulates the expression of MHC-I and CD95 target cells, enhancing the lytic and Fas-mediated death pathways.
|
|
Example of an integrated immune response, dermal infection:step1: innate immune detection of pathogen pattern (via TLR) activates Langerhans cells (dermal dendritic cells), which begins to produce cytokines and chemokines that 1. alter cell adhesion molecule expression on vascular endothelial cells (increased E-selectin expression) 2. enhance granulocyte extravasation
|
Step2: extravasation of granulocytes (neutrophils, monocytes) provides first response. Granulocytes bring to bear lytic enzymes and ROS poduction to manage pathogen invasion. Monocytes differentiate into active macrophages in reponse to local pro-inflammatory cytokine environment, proving additional support for killing and phagocytosis of pathogen and expanding the number of activated antigen presenting cells at the site.
|
|
STEP3: activated dermal dendritic cells migrate through lymphatic vessels to local lymph nodes. Upon arrival at the lymph node, fully matured APCs present pathogen peptides on MHC-II and activate cognate Th cells, Tc cells, and T memory cells (if not a first exposure). T-cells activated by antifen presentation begin to express CLA (PSGL-1) so that they are captured at the peripheral site of inflammation as they travel through the circulation. Capture is facilitated by upregulation of P-selectin on endothelial cells in response to local production of pro-inflammatory cytokines by macrophages, APCs, and fibroblasts.
|
Step4: Th and Tc cells that extracasate at peripheral sites of inflammation are further stimulated by to divide and enhance their state of activation by interactions with local APCs, generating a cascade of cellular and cytokine amplification.
|
|
Step5: activated Tc cells form immunological synapses with infected cells and kill if signaling is of appropriate strength, Activated Th1 cells form immunological synapses with local APCs (macrophages, Langerhans cells) and, if signaling is of appropriate strength, provide cytokine support for Tc cell amplification.
|
Step 6: Th2 cells activated peripherally or by interaction with APCs in the lymph node form immunological synapses with naive, pre-activated, or memory B cells. If signaling is of appropriate strength, the B-cell is driven to plasma cell fate which produces circulating antibody.
|
|
Step7: Circulating immunoglobulin enhances pathogen clearance by opsinization and complement fixation. Circulating immunoglobulin limits the ability of pathogen to move beyond initial site of inflammation.
|
|



