Cards In This Set
| Front | Back |
|
What is an example of a ionic and covalent bond?
|
NaCl is Ionic and H2O is covalent. Ionic is metal and non-metal generally, and covalent is 2 non-metals.
|
|
What is the lewis theory of bonding?
|
1) atoms and ions are stable if they have full valence shell of electrons
2) electrons are most stable when paired 3) atoms form chemical bonds to achieve a full valence shell of electrons 4) full valence shell of electrons can be done by sharing or transferring of electrons 5) sharing of electrons results in a covalent bond |
|
Draw the lewis structure, vsepr, and orbital diagram for HCN
|
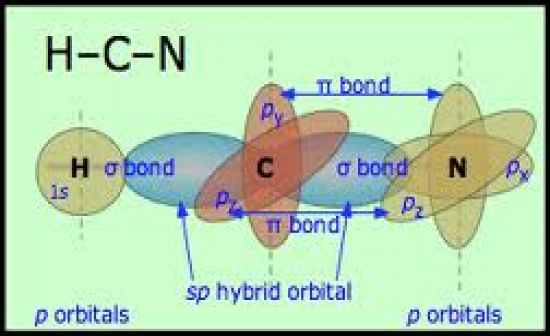 |
|
What is coordinate covalent bonding?
|
When one atoms donates 2 electrons in the bonding, not 1
|
|
What does the vsepr theory say?
|
(Valence Shell electron-pair Repulsion) theory states that you can determine the shape of an atom by minimizing the repulsive force between electron pairs and helps to determine the arrange of electron pairs
|
|
What is electron pair repulsion?
|
Bonded and lone pair electrons position themselves as far apart as possible in a molecule to minimize the repulsive force between them
|
|
How to determine whether the bond is polar?
|
EN <1.7
|
|
How to determine whether the bond is non-polar?
|
EN = 0
|
|
How to determine whether the bond is ionic
|
EN < 1.7
|
|
How do you whether molecular is polar or non polar
|
Add the vectors up, if there is a resultant, then the molecule is polar, if there isn't then the molecule is non- polar. If it is symmetrical then it is non-polar, vice versa.
|
|
What is the valence bond theory?
|
A covalent bond forms when 2 atomic orbitals overlap each other with an unpaired electrons- taking existing orbitals overlapping them to new molecular orbitals
|
|
What are hybrid orbitals?
|
They are formed when combining two different shaped orbitals, in a process called hybridization.
|
|
What are sigma bonds?
|
Sigma bonds are bonds that are head to head. Hybrid bonds can only form sigma bonds. They are single covalent bonds.
|
|
What are pi-bonds?
|
These orbitals overlap side by side which only occur in p-orbitals.
|
|
What is the difference between intermolecular and intramolecular
|
 Intermolecular is between molecules and intramolecular is within molecules. |



