Cards In This Set
| Front | Back |
|
7.1.1:
Describe a model of the atom that
features a small nucleus surrounded
by electrons.
|
Students should be able to describe a simple model
involving electrons kept in orbit around the nucleus
as a result of the electrostatic attraction between
the electrons and the nucleus.
· Nuclear model of atom has positively charged nucleus made up of protons and neutrons. · Negatively charged particles called electrons orbit the nucleus. · Most of the mass of the atom is concentrated in nucleus. · Electrons are much smaller than protons and neutrons. · Neutrons have no charge. · Neutral atoms are where the charges of the protons and electrons balance; same number of protons and electrons. |
|
7.1.2:
Outline the evidence that supports a
nuclear model of the atom.
|
A qualitative description of the Geiger–Marsden
experiment and an interpretation of the results are
all that is required.
· Alpha particles were shot at thin sheet of gold foil. · Most particles passed through, since atoms are made up mostly of empty space · Some particles deflected at large angles, indicating a solid, positively charged structure within atoms. |
|
7.1.3: Outline one limitation of the simple
model of the nuclear atom.
|
Limitations
include: the model does not take into consideration isotopes and different
atomic weights, and the model does not show how the atom would be stable.
Electrons would lose energy with radiation.
|
|
7.1.4:
Outline evidence for the existence of
atomic energy levels.
|
Students should be familiar with emission and
absorption spectra, but the details of atomic
models are not required.
Students should understand that light is not a
continuous wave but is emitted as “packets” or
“photons” of energy, each of energy hf.
Absorption and Emission Spectra provide evidence for energy levelsSpectra of each element is uniqueElectrons can only occupy specific energy levels.Movement between energy levels requires electron to emit or absorb energy.Energy emitted or absorbed is in the form of packets of light called photons.E=hf (Energy of a photon = Planck’s constant*frequency of light in Hz).Energy is "quantized". |
|
7.1.5:
Explain the terms nuclide, isotope and
nucleon.
|
1.
Nuclide – a
particular type of nucleus with a certain number of protons and neutrons
2.
*Isotope - nuclei
with the same number of protons (Z) but different number of neutrons (N)
3.
*Nucleon – a proton or
neutron (NOTE: Do not say “a
particle in the nucleus” since that would include quarks as well.)
|
|
7.1.6:
Define nucleon number A, proton
number Z and neutron number N.
|
1.
Nucleon Number (Mass Number) (A) - number of nucleons (protons + neutrons) in nucleus
2.
*Proton Number (Atomic Number) (Z) - number of protons in nucleus
3.
*Neutron Number (N) - number
of neutrons in nucleus (N = A – Z)
|
|
7.1.7:
Describe the interactions in a nucleus.
|
Students need only know about the Coulomb
interaction between protons and the strong,
short-range nuclear interaction between nucleons.
Coulomb Force: The positively charged protons repel one another. Nucleons are arranged in such a way that like charges repel as far as possible. Electrostatic force of repulsion between the protons in the nucleus. Strong Nuclear Force: Strong force of attraction between nucleons that overcomes Coulomb repulsion between protons. Force is independent in whether particles are neutrons or protons. At 1.3 femtometers, force is about 100 times stronger than repulsion forces; if the distance between protons is greater than 1.3 fm, force falls to 0, if the distance is smaller than 1.3 fm, strongly repulsive. |
|
7.2.1: Describe the phenomenon of natural
radioactive decay.
|
When an
unstable nucleus emits a particle (alpha, beta, gamma) (NOTE:
Radioactive decay is both a random
and a spontaneous process.) (NOTE:
The rate of radioactive decay decreases exponentially with time.)
Process is independent of pressure, temperature, and chemical combination. |
|
7.2.2:
Describe the properties of alpha (α)
and beta (β) particles and gamma
(γ) radiation.
|
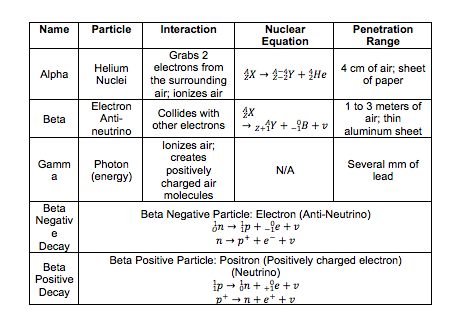 See image. |
|
7.2.3:
Describe the ionizing properties of
alpha (α) and beta (β) particles and
gamma (γ) radiation.
|
· When alpha particles pass through air, they take two
electrons from the air molecules, and become neutral helium atoms.
· Air molecules are left with a charge i.e. the air is
ionized.
· Beta particles and gamma radiations may also cause
ionization; the particles of the radiation remove electrons, creating electron
ion-pairs.
|
|
7.2.4:
Outline the biological effects of
ionizing radiation.
|
Students should be familiar with the direct and
indirect effects of radiation on structures within
cells. A simple account of short-term and long-term
effects of radiation on the body is required.
· Radioactive radiations can cause severe damage to living organisms. · May affect DNA and genetic coding of future generations. · Probability of genetic damage increases with increasing intensity and exposure to radiation. · Illnesses such as leukemia and cancer can result from exposure; even death. |
|
7.2.5:
Explain why some nuclei are stable
while others are unstable.
|
An explanation in terms of relative numbers of
protons and neutrons and the forces involved is all
that is required.
· For elements with Z less than about 20, the protons and neutrons are in equal numbers. · Due to an increase in the electrostatic repulsion forces of protons as the number of protons increases, more neutrons must be found in nucleus to hold atom together. · Each time protons and neutrons are added, they must go into higher energy state, and eventually become unstable. · Unstable nuclei emit alpha particles (two protons and two neutrons) in order to reach a more stable state. |
|
7.2.6:
State that radioactive decay is a
random and spontaneous process
and that the rate of decay decreases
exponentially with time.
|
Exponential decay need not be treated analytically.
It is sufficient to know that any quantity that
reduces to half its initial value in a constant time
decays exponentially. The nature of the decay is
independent of the initial amount.
|
|
7.2.7:
Define the term radioactive half‐life.
|
The time taken for ½ the number of radioactive
nuclei in sample to decay .
OR The time taken for the activity of a sample to decrease to ½ its initial value. |
|
7.3.1:
Describe and give an example of an
artificial (induced) transmutation.
|
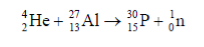 Artificial Transmutation: When a nucleus is bombarded with a nucleon, an alpha particle or another small nucleus, resulting in a nuclide with a different proton number (a different element). · Can also be used to form radioactive isotopes of elements. · Neutrons are effective in inducing artificial transmutation to form artificial radioactive isotopes. |



