Cards In This Set
| Front | Back |
|
Use of different media
|
Selective
• Select for specific types of bacteria
- Chemicals inhibit one group but allow another to grow
• PEA agar, CV agar, NaCl agar
Differential
• Distinguish among morphologically & biochemically
related groups of orgs.
- specific characteristics on media
• MSA, MacConkey, EMB
Enriched
• Rich media for fastidious orgs.
- Contains blood, serum, or yeast extract
• Blood agar, chocolate agar
|
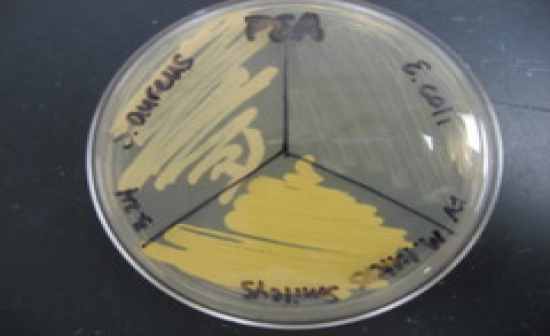 Phenylethyl Alcohol Agar (PEA) |
Selects for G+ orgs
• Inhibits most G- orgs.
• Must compare growth to
a control plate (EMB,
MAC, BHI or TSA)
Results –
• Growth compared to
control = (+)
• No growth or Inhibited
growth = (-)
NA PEA
|
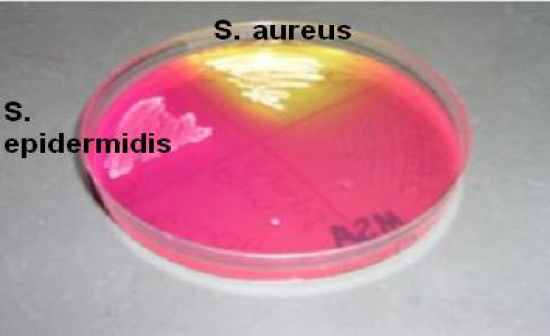 Mannitol Salt Agar (MSA) |
Differential & selective
• Selects for halophiles with high [salt] – 7.5% NaCl
• Differentiates based on ability to ferment mannitol
pH indicator = phenol red
• Yellow = acid production = + fermentation
Results
• Growth or no growth (inhibited)
• A= or Ahttp
|
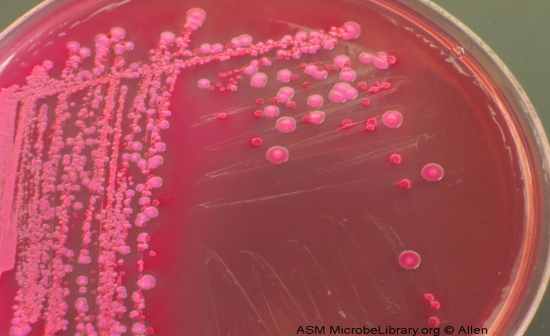 MacConkey Agar (MAC) |
Selective
• Selects for Gram (-) – Crystal violet & bile salts inhibit G(+)
Differential
• Ability to ferment lactose
• pH indicator = neutral red, turns pinkish/red when pH < 6.8
Results – Compare to PEA for control
• Orgs that ferment lactose red growth (Differential)
• Non-fermenters produce non-colored colonies
• Inhibited/No growth = (-) (Indicates G+ organism)
|
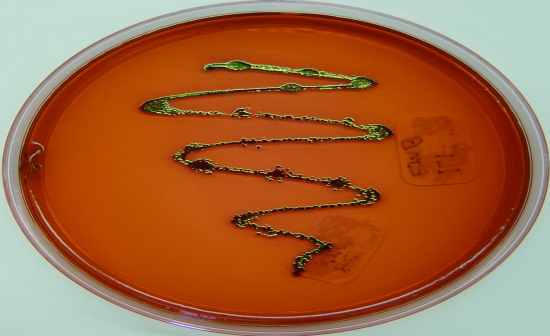 Eosin-Methylene Blue (EMB) |
Selective & differential media
• Eosin & MB inhibit G+ bacteria
• Differentiates based on ability to ferment lactose &/or sucrose – dyes interact
to change the color of growth
• Used for isolation of fecal coliforms
Results:
• Inhibited / no growth = (-) Indicates G+ organism
• Good growth = indicates G- organism
- Green sheen / black = vigorous fermenter (E.coli or C. freundii)
- Pink/Purple = less aggressive lactose fermenter (Enterobacter or Klebsiella)
- No color (creamy/white/same color as agar) = non lactose fermenter or possible
sucrose fermenter
|
 Oxidation-Fermentation Test |
Two tubes –
• One covered w/ mineral oil to promote anaerobic growth & fermentation
• Mineral oil prevents oxidation
Results
• Ferment & oxidize - sealed & unsealed yellow throughout
• Oxidize only - unsealed broth yellow; sealed staying green
• Slow or weak fermenters both tubes slightly yellow in top
• Non-saccharolytic both green or blue (strictly aerobic)
|
|
Fermentation of Sugars
|
Conversion of sugar
to acid, alcohol & gas
(CO2)
Phenyl red broth
• PR –ose
Phenyl red = pH
indicator
• Acid = yellow
• Neutral = red
• Alkaline = hot pink
|
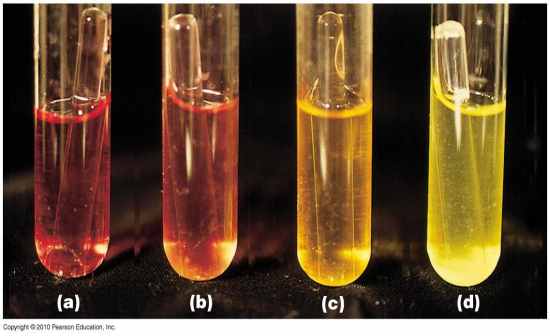 PR Carbohydrate Broth |
CHO fermentation = yellow (acidic) = A+
CO2 production trapped in Durham tube = Gas +
Hot pink = degradation of proteins/AA NH3 (alkaline) = K+ or P+
|
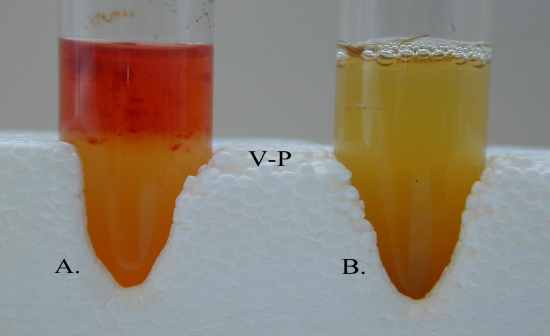 MR-VP |
Methyl Red & Vogues
Proskauer Tests
Low acid fermentation product
is maintained by E.coli
• E.aerogenes converts acids to
non-acidic end products
(acetoin & 2,3-butanediol)
which raise pH to ~6
|
 Procedural Diagram for MR-VP Tests |
After growth place split into
two tubes
• To one tube add ~4-5 drops
MR (+ if turns red)
• To other tube add 15 drops
VP-A (Barritt’s) & 5 drops
VP-B – wait 60 minutes
|
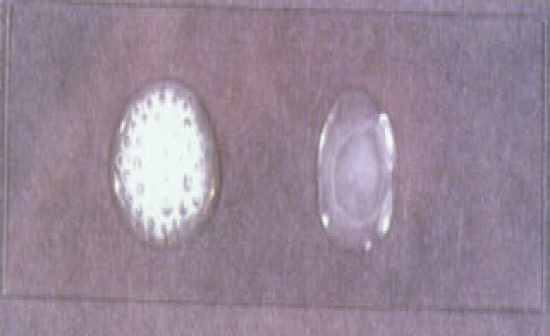 Catalase Test |
Presumptive test - slide test
• Drop DI H2O on one side &
drop of peroxide on the other
• Add 1 drop of culture to each
spot
- If bubbles = (+) for catalase
Confirming test
• Add 1-5 drops H2O2 to culture
growing on slant
- (+) = bubbles
|
 Oxidase Reaction |
Use wood end of cotton applicator place on DrySlide – read at 20 seconds
Add 1-3 drops oxidase reagent to slant
• (+) = purple/black
|
 Nitrate Reduction |
Tests for nitrate reductase
Air bubble in Durham tube
indicates gas production =
denitrification
If a non-fermenter test is complete
= NO3 NO2 N2
If no gas or a fermenter add
reagents
• Add 8 drops Nitrate A & B reagent
Results
• Immediate red = (+)
• If colorless add zinc
- Remains colorless = (+)
- Turns red = (-)
|
 Citrate Test - Simmons Citrate Agar |
Tests for citrate permease
• Citrate as only C source
• pH indicator = bromthymol blue
- Green pH 6.9 and blue at pH > 7.6
Inoculate w/ needle (light inoculum)
• (+) if media turns royal blue
• Growth no color change = (+) (rare – make sure not heavy inoculum)
• No growth / No color change = (-)
|
 Decarboxylation test |
Mineral oil promotes
fermentation
Decarboxylation of AA
alkaline products (purple) = +
If a glc fermenter but does
not have decarbixylase =
yellow (-)
No color change = (-)
|



