Cards In This Set
| Front | Back |
|
7.1.1: Describe a model of
the atom that features a small nucleus surrounded by electrons
|
·
Nuclear model of atom has positively charged
nucleus made up of protons and neutrons.
·
Negatively charged
particles called electrons orbit the nucleus.
·
Most of the mass of the atom is concentrated in
nucleus.
·
Electrons are much smaller than protons and
neutrons.
·
Neutrons have no charge.
·
Neutral atoms are where the charges of the
protons and electrons balance; same number of protons and electrons.
|
|
7.1.2: Outline the evidence
that supports that nuclear model of the atom.
|
·
Geiger Marsden / Gold Foil Experiment
·
Alpha particles were shot at thin sheet of gold
foil.
·
Most particles passed through, since atoms are
made up mostly of empty space
·
Some particles deflected at large angles,
indicating a solid, positively charged structure within atoms.
|
|
7.1.3: Outline one
limitation of the simple model of the nuclear atom.
|
Limitations include: the model does not take into
consideration isotopes and different atomic weights, and the model does not
show how the atom would be stable. Electrons would lose energy with radiation.
|
|
7.1.4: Outline evidence for
the existence of atomic energy levels.
|
·
Atomic energy levels are distinct regions around
the nucleus where electrons are likely to be found.
·
As electrons gain energy (become excited), they move
up to energy levels farther away nucleus.
·
When electrons return to their ground state
(initial energy level), they emit the energy gained in the form of packets of
light called photons.
·
E=hf (Energy of a photon = Planck’s
constant*frequency of light in Hz)
·
Absorption and Emission Spectra provide evidence
for energy levels.
|
|
7.1.5: Explain the terms
nuclide, isotope, and nucleon.
|
Nuclide: A particular type (species) of nucleus with a certain number of
protons and neutrons.
Isotope: Nuclei with the same number of protons (Z) but different number of
neutrons (N) e.g. C-12 and C-14.
Nucleon: A proton or neutron (not necessarily a particles within nucleus).
|
|
Topic 7.1.6: Define nucleon
number A, proton number Z, and neutron number N.
|
Nucleon Number: Number of nucleons (Protons + Neutrons) in
the nucleus. Also known as mass number. Symbol is A.
Proton Number: Number of protons in the nucleus . Also
known as atomic number. Symbol is Z.
Neutron Number: Number of neutrons in the nucleus. Symbol
is N.
N=A-Z
|
|
7.1.7: Describe the
interactions in a nucleus
|
Coulomb Force:
·
The
positively charged protons repel one another.
·
Nucleons
are arranged in such a way that like charges repel as far as possible.
·
Electrostatic
force of repulsion between the protons in the nucleus.
Strong Nuclear
Force:
·
Strong
force of attraction between nucleons that overcomes Coulomb repulsion between
protons.
·
Force is
independent in whether particles are neutrons or protons.
·
At 1.3
femtometers, force is about 100 times stronger than repulsion forces; if the
distance between protons is greater than 1.3 fm, force falls to 0, if the
distance is smaller than 1.3 fm, strongly repulsive.
|
|
7.2.1: Describe the
phenomenon of natural radioactive decay
|
·
Radioactivity
is when elements emit radiation spontaneously.
o An unstable nucleus emits a particle (alpha,
beta, or gamma).
·
Radiation
is independent of pressure, temperature, and chemical combination.
·
Radioactive
properties caused by nucleus of atoms.
·
The rate
of radioactive decay decreases exponentially with time.
|
|
7.2.2: Describe the
properties of alpha and beta particles and gamma radiation
|
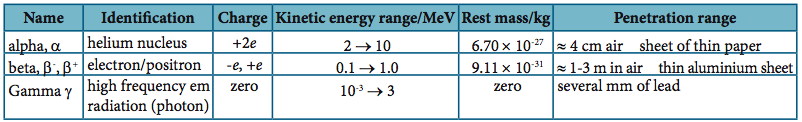 Refer to image |
|
7.2.3: Describe the ionizing
properties of alpha and beta particles and gamma radiation.
|
·
When
alpha particles pass through air, they take two electrons from the air
molecules, and become neutral helium atoms.
·
Air
molecules are left with a charge i.e. the air is ionized.
·
Beta
particles and gamma radiations may also cause ionization; the particles of the
radiation remove electrons, creating electron ion-pairs.
|
|
7.2.4: Outline the
biological effects of ionizing radiation.
|
·
Radioactive
radiations can cause severe damage to living organisms.
·
May
affect DNA and genetic coding of future generations.
·
Probability
of genetic damage increases with increasing intensity and exposure to
radiation.
·
Illnesses
such as leukemia and cancer can result from exposure; even death
|
|
7.2.5: Explain why some
nuclei are stable while others are unstable.
|
·
For
elements with Z less than about 20, the protons and neutrons are in equal
numbers.
·
Due to
an increase in the electrostatic repulsion forces of protons as the number of
protons increases, more neutrons must be found in nucleus to hold atom
together.
·
Each
time protons and neutrons are added, they must go into higher energy state, and
eventually become unstable.
Unstable nuclei emit alpha particles (two protons and two neutrons) in
order to reach a more stable state
|
|
7.2.7: Define the term
radioactive half-life.
|
Radioactive
Half-Life (Two Definitions):
·
The time
taken for half the number of radioactive nuclei in a sample to decay.
·
The time
taken for the activity of a sample to decrease to half its initial value.
|
|
7.3.1: Describe and give an
example of an artificial (induced) transmutation.
|
Artificial Transmutation: When a nucleus is bombarded with a
nucleon, an alpha particle or another small nucleus, resulting in a nuclide
with a different proton number (a different element).
·
Can also be used to form radioactive isotopes of
elements.
Neutrons are effective in
inducing artificial transmutation to form artificial radioactive isotopes
|
|
7.3.3: Define the term
unified atomic mass unit
|
Unified Atomic Mass
Unit: 1/12th the mass of a Carbon-12 nucleus.
·
Units
created to compare atomic masses, since individual masses in nuclear reactions
are very small.
·
The mass
one proton (or neutron) are approximately 1 u.
|



