Cards In This Set
| Front | Back |
|
Trend in size
|
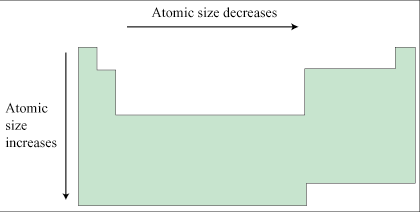 As you move left, it increases as you move down, it increases |
|
Trend in electron affinity
|
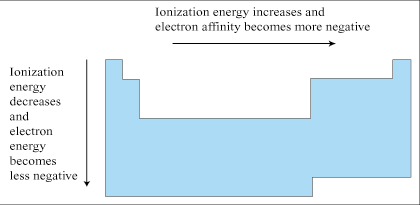 (look at pic) |
|
Trend in ionization energy
|
As you move left it decreases,
as you move down, it decreases |
|
Trend in metallic behavior
|
As you move left, it increases
as you move down, it increases |
|
Trend in ionic size
|
As you move right, it increases
as you move down, it increases |
|
Ionization energy
|
Energy required to remove 1 mol of electrons
|
|
Electron affinity
|
The energy change in the addition of 1 mol of electrons to 1 mol of gaseous atoms/ions
|
|
Paramagnetism
|
A species with unpaired electrons
|
|
Diamagnetism
|
A species with paired electrons
|
|
Electronegativity (E)
|
Ability of an atom to attract electrons towards itself in a covalent bond
|
|
E of F
|
4
|
|
E of O
|
3.5
|
|
E of Cl
|
3
|



